A cursory search through the internet reveals countless alarming articles on antibiotic resistance: “Drug-resistant superbug a factor in seven deaths in Limerick”. “Nevada Woman Died From Near-Ultimate Superbug”. “‘Antibiotic apocalypse’: doctors sound alarm over drug resistance”, “A Superbug That Resisted 26 Antibiotics.” According to a 2013 CDC report on antibiotic-resistant threats to the US, more than two million people contract antibiotic-resistant infections every year and at least 23,000 die as a result. This data is based off conservative current-day estimates: projections for the future suggest that by 2050, 10 million mortalities a year will result from antibiotic-resistant bacteria, leading to a 2% to 3.5% reduction in GDP and costing the world up to 100 trillion USD, surpassing mortality via cancer, diabetes, and road traffic accidents. The chief medical officer of England, Professor Dame Sally Davies, has stated that antibiotic resistance “poses a catastrophic threat,” and that if it progresses further, “anyone…could go into hospital in 20 years for minor surgery and die because of an ordinary infection that can’t be treated by antibiotics”.
Despite these bone-chilling predictions, antibiotic resistance is a little-understood phenomenon and little has been done to help curb its rise. Scant effort is put into developing new varieties of antibiotics and new therapies face immense hurdles before reaching market viability. Antibiotic resistance does not have the widespread publicity that diseases such as cancer or tetanus do, and consequently, less funds are allocated for research in this field. Overuse of current antibiotics in many industries creates a favorable environment for new varieties of antibiotic resistant bacteria to evolve, allowing for more lethal varieties to sprout up with time. As a result, the future antibiotic resistance continues to look grim.
History of Antibiotic Use
In August 1928, Sir Alexander Fleming had returned to his laboratory to see a mold colony in a Staphylococcus bacterial petri dish. Interestingly, there was a halo around the mold, within which the bacteria did not form colonies. Suspecting an antibacterial compound, Fleming began further investigating the mold, Penicillium notatum, and the compound eventually isolated was later named penicillin in reference to the mold.
The discovery of penicillin and its antibacterial properties revolutionized the medical world. The compound was used widely among Allied forces during WWII, and became available for public use in 1944. Penicillin revolutionized public health: Minor injuries no longer carried the threat of fatal illness, and previously lethal infections became nothing more than a few days of discomfort. However, upon receiving the 1945 Nobel Prize in medicine and physiology, Fleming ominously warned that “it is not difficult to make microbes resistant to penicillin in the laboratory by exposing them to concentrations not sufficient to kill them.”
Regardless, research into antibiotics pressed forward. In 1948, animal nutritionist Robert Stokstab and biochemist Thomas Jukes discovered that the cellular remains generated by antibiotic production allowed chicks to grow 24 percent faster while costing much less than traditional feed supplements. In the 1950s to the 1970s, large amounts of new antibiotics were developed, treating diseases ranging from meningitis and syphilis. Today, antibiotics are of critical importance in surgery, dialysis, cancer chemotherapy, inflammatory conditions, and organ transplants, and are foreseen to increase in importance as future medical technologies rise.
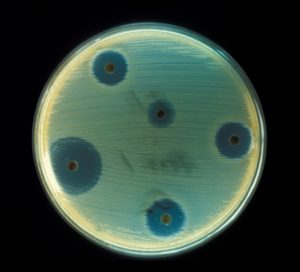
Currently, there are only five ways that an antibiotic kills bacteria. The first antibiotics killed bacteria by interfering with cell wall synthesis. Currently, most antibiotics interfere with protein synthesis, and antibiotics such as chloroquine inhibit enzyme functions within the bacterium. Some antibiotics interfere with DNA synthesis, and some still interfere with RNA synthesis. Antibiotics are also frequently used as anti-cancer agents. No new discoveries on other antibiotic action pathways have been made and there is very little financial incentive to do so: The gargantuan market demand for current antibiotics encourages the continued production of traditional antibiotics and discourages financially costly research not directly involved in generating market profits.
True to Alexander Fleming’s word, bacterial resistance to penicillin began to grow. By 1955, the spread of penicillin resistance prompted nations around the world to restrict penicillin use to prescription only. Unfortunately, this legislation did not successfully suppress increasing penicillin resistance. As a response, scientists developed methicillin. Methicillin was primarily used to treat staph infections caused by Staphylococcus aureus, a bacterium which can lead to skin lesions, digestive upsets, and more seriously, meningitis, toxic shock syndrome, and sepsis. Initially, the new antibiotic seemed to resolve the problem of penicillin resistance, but in 1961, British scientists discovered Methicillin-resistant Staphylococcus aureus, or MRSA, and in 1968, MRSA spread to the United States. In the 1990s, a stronger strain of MRSA developed resistance to multiple antibiotics and spread beyond the hospital. Now, antibiotic resistant bacteria endangered the general populace, and by 2002, up to 60 percent of S. aureus infections were deemed to be methicillin resistant.
While humans have been discovering new antibiotics, evolution currently has the supper hand: In 2010, a study on a species of Enterobacteriaceae bacteria, a bacterial family which includes Salmonella, E.coli, and Yersinia pestis–the bacterium responsible for causing the Black Death–discovered it to be resistant to carbapanems, the most powerful type of antibiotic currently available. Evolution is rapidly outpacing the speed of human innovation, and this creates for the dangerous situation humanity is currently facing.
Agriculture and Antibiotic Resistance
The vast majority of market demand for antibiotics comes from agriculture. In 2014, pharmaceutical companies sold nearly 21 million pounds of medically important antibiotics for animal usage, a figure that dwarfs the amount in use for humans by threefold. The high density of intensively-managed, genetically-homogenous animals in industrial agriculture requires bacterial control, and growth-promoting factors in antibiotics further incentivize its mass usage in agriculture. Industrial animals are often raised on diets that contain antibiotics, and the continual exposure of farm animals to antibiotics gives bacteria the time to evolve mechanisms for resistance.
Eerily, discoveries have shown that antibiotic-resistance genes can be passed around among bacteria. Bacterial genes can either be found on the nucleoid DNA cluster in the center of the bacterial cell, or on plasmids, circular loops of DNA that float around in the bacteria cell. These plasmids can be traded and replicated between different bacteria, allowing a gene to quickly spread among a bacterial population. Unfortunately, genes encoding for antibiotic resistance are most often found on these plasmids.
In factory farms, it only takes a single bacteria that has developed antibiotic resistance to lead to an outbreak of antibiotic-resistant bacteria, and antibiotic-resistant bacteria can even jump between different species. A 1975 study conducted by Tufts University biologist Stuart Levy under the Animal Health Institute brought this phenomenon to the public light. Levy fed low doses of the antibiotic tetracycline to a group of 150 chickens that had never been exposed to antibiotics previously. After a week, most of the E. coli bacteria found within the chicken’s digestive system were found to have tetracycline-resistance, and after three months, other species of bacteria nearby also developed resistance. Fourth months into the experiment, the neighbouring chickens were also found to have antibiotic resistant bacteria, and the farm owners were found with resistant bacteria as well. With this ripple effect of antibiotic usage, it is no surprise why farms are such a rich breeding ground for antibiotic resistance. Agricultural practises that use the manure of animals in fertilizing plants allows antibiotic-resistant bacteria to expand beyond the meat-production environment and into plant agriculture as well. Coupled with the usage of antibiotics in plant cultivation, vegetarians and vegans have also been found with antibiotic resistant bacteria in their systems, showing how antibiotic resistance is not simply an issue for certain demographics, but a danger to us all.
New Solutions
Given the dire circumstances surrounding increasing mortality from antibiotic-resistance bacteria, new solutions are urgently needed to ensure the health and safety of humanity for years to come. State agencies such as the CDC have focused on investing in containment infrastructure in the event of an antibiotic-resistant infection, preparing for quick responses in case of an outbreak, and increasing legal regulation of antibiotics. Without exposure to antibiotics, antibiotic-resistant bacteria naturally lose their plasmids, and with time, antibiotic-resistance can diminish in a bacterial population.
However, reducing antibiotic usage in agriculture and in hospitals to fully eliminate antibiotic resistance is highly implausible, not to mention morally questionable. A new solution, however, is emerging on the market: Bacteriophage usage.
Bacteriophages, or phages for short, are viruses that specialize in attacking bacteria. In the wild, bacteriophages will inject their DNA or RNA into a bacterial cell, hijacking the cell’s gene expression mechanism to prompt it to replicate phages to the point of bacterial death. Like many antibiotics, bacteriophages are equipped to attack bacteria only. Unlike antibiotics, phages can evolve new proteins, allowing for infinite possibilities of designing the phage and of tackling the resistant bacteria and making it harder for the bacteria to evolve resistance to the treatment.
Research into phage therapy was initially pioneered in the former Soviet Union nearly a century ago and is still widely used today in Russia, Georgia, and Poland. Despite the promise of this new solution, barriers still loom for phage therapy as a replacement to antibiotics. A major issue is survival of the phages: Unlike antibiotics, phages are living organisms that are adapted to certain environments, making it difficult to ensure the phage remains alive when it reaches the disease site. Also, intellectual property laws surrounding phage therapy are murky due to the long history of this treatment, which makes it difficult for legislators to pinpoint how the treatment originated and which makes patenting the process extremely difficult. While many studies have shown more than satisfactory results, some point to difficulties in targeting certain bacteria or result differences when phage therapy is applied to different species.
Regardless of which solution to take, however, time is running out. As the death toll from antibiotic resistant infections increases, science must produce a reliable way to target antibiotic resistance, be it through encouraging more precise regulation or inventing a more innovative solution. However, given the acceleration of scientific breakthroughs in the recent decade – most notably in the field of biology – humanity has every reason to remain optimistic and to believe that the projected 10 million mortalities from antibacterial resistance is, in the end, just a projection.
Image Credit: Wikimedia Commons/Don Stalons